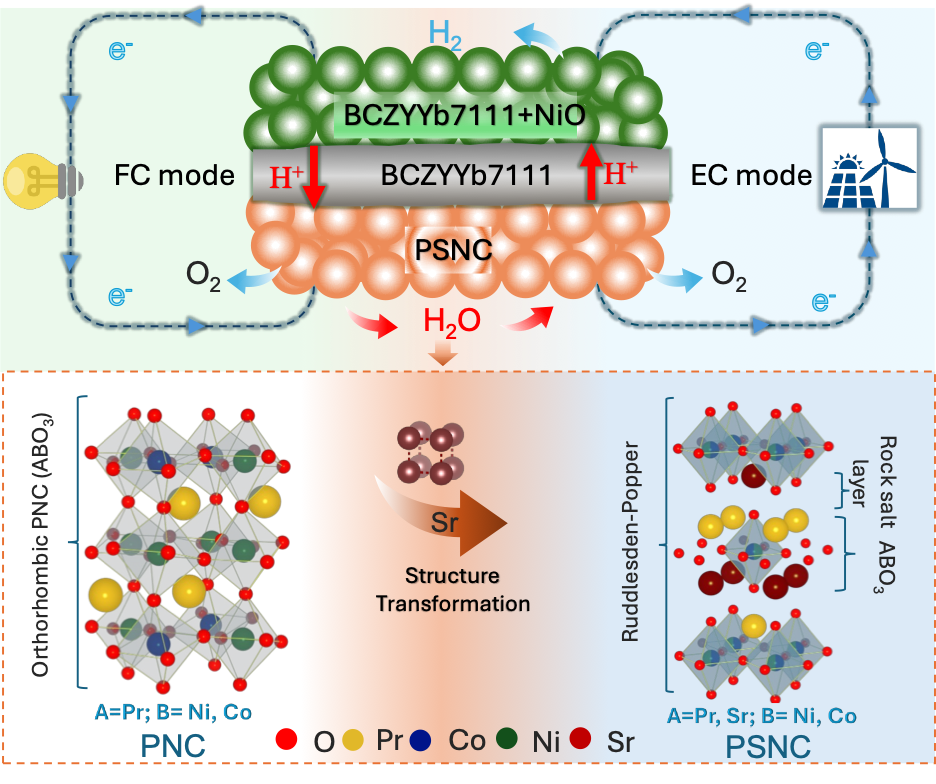
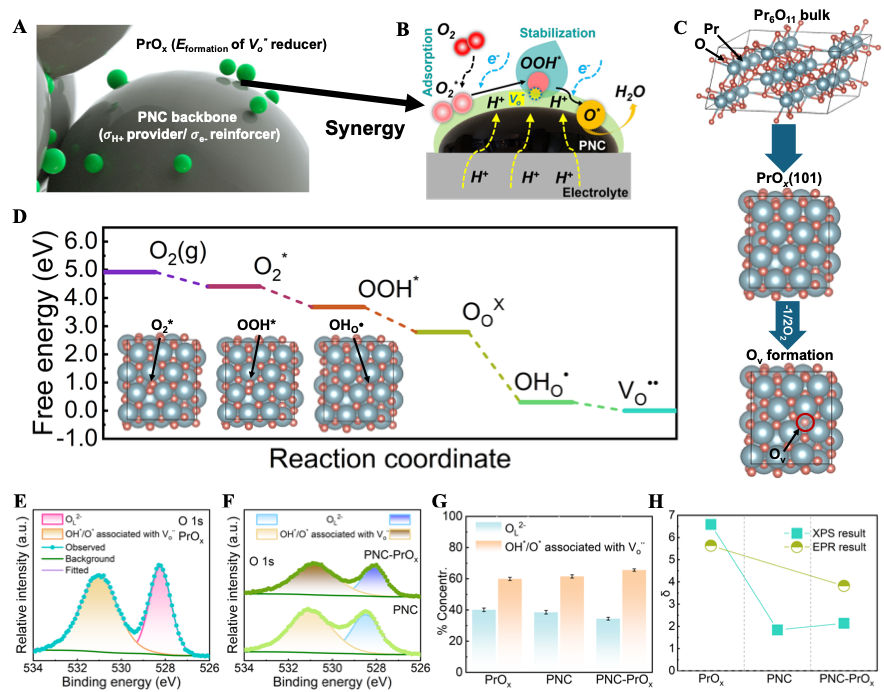
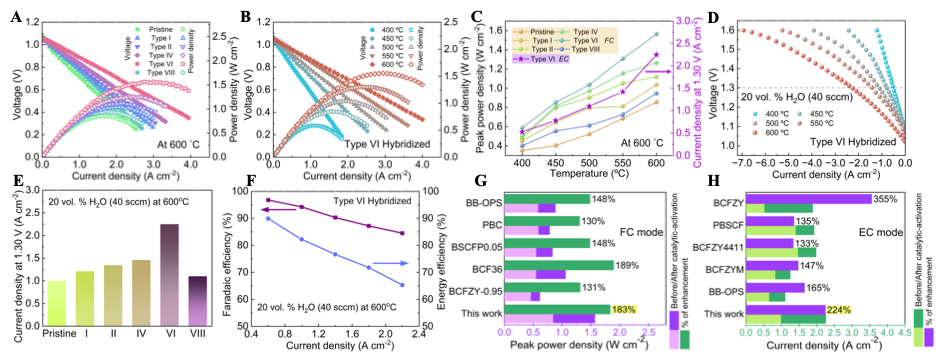
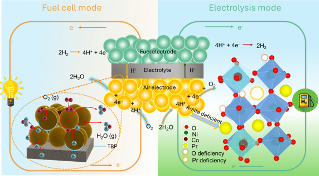
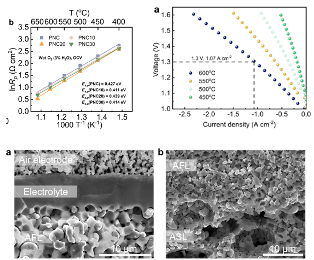
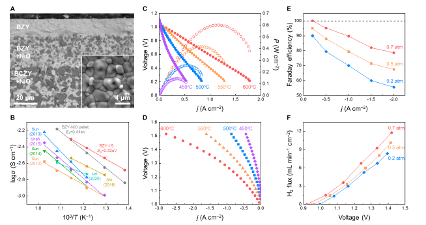
We recently have a work “Structural Transformation of Oxygen Electrode from Perovskite to Ruddlesden-Popper for Enhanced Reversible Hydrogen Production and Power Generation in Protonic Ceramic Cells” accepted by Materials Today (IF=22). In this work, we a novel Ruddlesden-Popper (R-P) structured electrode, (Pr0.6Sr0.4)2Ni0.7Co0.3O4+δ (PSNC), produced by systematic strontium doping in PrNi0.7Co0.3O3-δ (PNC) for intermediate-temperature reversible protonic ceramic electrochemical cells.
The strategic Sr2+ substitution for Pr3+ causes a structural transition from an orthorhombic perovskite to a layered R-P phase, generating well-defined routes for improved ionic transport. Electrochemical characterizations reveal outstanding bifunctional performance, with the PSNC electrode obtaining a peak power density of 1.03 W cm-2 in fuel cell mode and a current density of 1.30 A cm-2 at 1.30 V in electrolysis mode at 600 °C. The cell demonstrates exceptional operational resilience and mechanical-electrochemical robustness, maintaining long-term stability despite vigorous dynamic voltage cycling. Faradaic efficiency experiments at 1.16 V under 50% steam show up to 85% efficiency and highly steady extended galvanostatic operation up to 2.0 A cm-2, indicating the electrode’s durability and stability in harsh environments. Structural and interfacial investigations confirm the electrode’s pristine integrity and high compatibility with the electrolyte. These synergistic properties position PSNC as a promising choice for next-generation energy conversion devices, allowing for seamless transitions between power generation and hydrogen production under realistic conditions.
The paper link will be shared after publishing online.